Realize a greater return on your initial investment by focusing on the high-impact capabilities your company needs at this stage of clinical drug development, implementing a pre-validated, turnkey solution in weeks, not months, and establishing a strong regulatory foundation that will yield future savings and support growth.
LifeSphere Investigational Product allows you to:
- Comply with global regulatory and international product data standards from the start
- Align your authoring plan with your regulatory submission outline directly in RIMS
- Improve cross-functional and third-party collaboration
- Effectively manage and track health authority commitments
- Step into powerful functionality like global product registration tracking when you’re ready
Discover how LifeSphere Investigational Product RIMS adds value
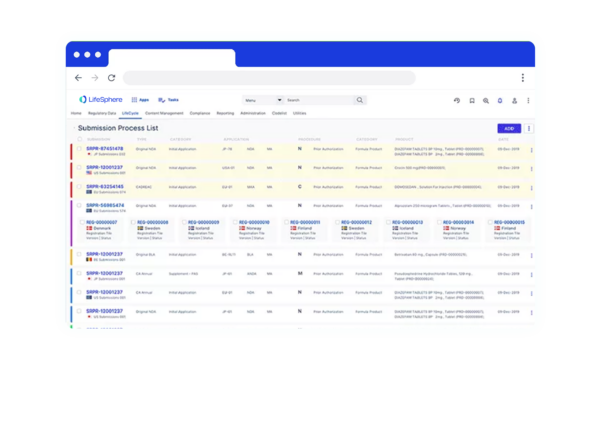
Enable more effective collaboration
Cross-functional teams have 360° visibility to project- and task-level deadlines and status updates through dashboards and reporting in a single interface. Additionally, interoperability with third-party technologies promotes collaboration with partners like Contract Research Organizations (CROs).
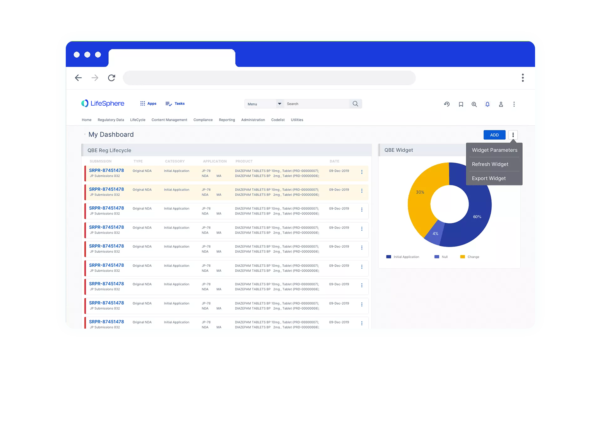
Speed to value
Focus on what’s right in front of you with purpose-built functionality to lower initial costs, a pre-validated, turnkey solution that can be implemented in weeks, not months, and a renewable annual contract term that gives you the ability to convert a short-term investment into sustained success.
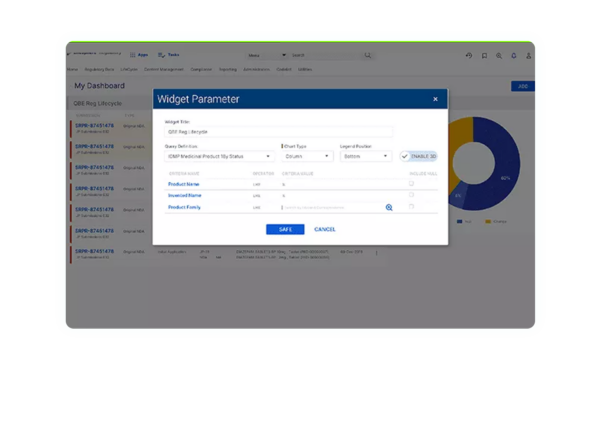
Investor-ready product assets
By establishing compliance with global data standards and naming conventions for the exchange of information and storing approved investigational submission content in a validated system, investigational-stage companies can present a more polished package of product assets to potential investors and commercialization partners.
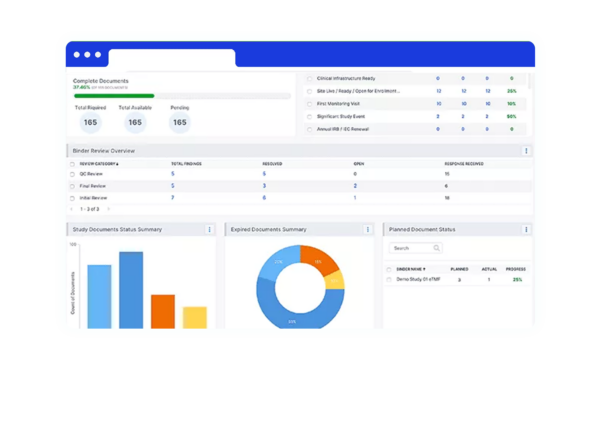
Condensed marketing authorization timeline
As you receive the green light from health authorities, quickly repurpose approved investigational submission content for your marketing applications worldwide.

Step into robust functionality
Harness the full power of LifeSphere Regulatory by simply upgrading your license to step into the more complex capabilities needed to manage marketing authorizations and track global product registrations. There’s no need to migrate data, rebuild workflows and import data standards.
Seeing is believing
Schedule a LifeSphere Regulatory Investigational Product RIMS demo to see how functionality is tailored to the needs of life sciences companies working through the clinical phases of drug development.
Purpose-built for investigational-stage companies
LifeSphere Investigational Product is purpose-built with the functionality you need today, nothing more. Organizational complexity and cost are reduced by focusing on high-priority capabilities like investigational submission and dossier planning, clinical trial authorization tracking, and health authority commitment management.
Investigational submission and dossier planning
with precision automation and activity triggers to drive efficiency
Clinical trial authorization tracking
to meet regulatory deadlines and expedite time to market
Health authority commitment management
for end-to-end planning and tracking of interactions, commitments, and obligations
Don’t Take Our Word for It, Here’s What Industry Voices Say about LifeSphere Regulatory
No matter your size, your organization is in good company. LifeSphere Regulatory services over 30 customers worldwide, including 4 of the top 20 life sciences organizations. Validated by industry-leading voices, your organization can trust that LifeSphere Regulatory provides modern solutions that are forward-thinking and ready to grow with your organization
Gens & Associates IDMP Readiness Review
LifeSphere Regulatory IDMP is ranked number one in IDMP readiness out of all vendors on this review ahead of the 2023 implementation deadline and worked within the industry to enable customers with internal experts.
Frost & Sullivan Customer Value Leadership
LifeSphere solutions in both Regulatory and Clinical are recognized for their customer value in North America as a cloud-enabled, data-first customer solution in Life Sciences.
Resources for Your Regulatory Journey
Checklist: What to Look for in Your Investigational Product RIMS Solution
Investigational-stage firms that invest in their own RIMS solution early on can drive efficiencies, ensure future market readiness, and adapt to changing regulatory requirements.
Webinar: 'Right-Sized' RIMS
Join ArisGlobal leaders to discover how a regulatory information management solution tailored to investigational-stage company needs can reduce complexity and provide immediate value.
Fact Sheet: LifeSphere Investigational Product
Purpose built RIMS with the critical functionality life sciences companies and their partners need during the clinical phases of drug development.
Learn more about our collaborative partnership with customers

