Enable your organization
Elevate your organization’s technology to enable more collaboration and better outcomes.
Grow your organization with a scalable platform that evolves to meet your changing needs.
Maintain control of all your data with an in-house, efficient system.
Maximize efficiency with industry standards and robust APIs that ensure seamless integrations.
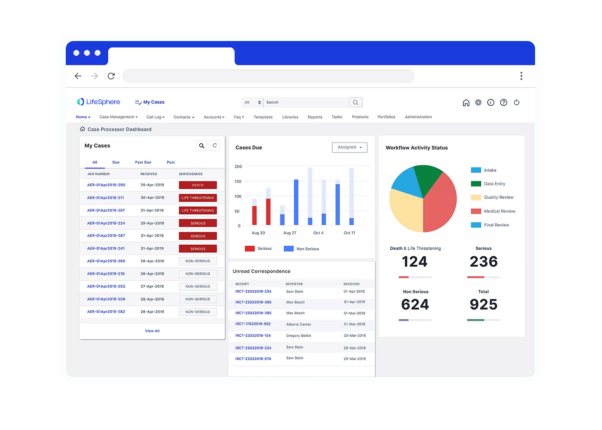
LifeSphere Solutions for Small to Medium Pharma
Modernize your pharmacovigilance functions with the Safety platform trusted by over 300 life science firms
Keep your Safety team lean with the only end-to-end, production-ready, automated case management solution.
Maximize partner flexibility and oversight with a single global platform with central dashboards and reports.
Future-proof your compliance with a platform that scales to any case volume and global operation.
Ensure compliance with continually updated cloud software trusted by more than 30 leading life science organizations.
Deploy intelligent automation to reduce workload and free resources for more cognition-intensive tasks.
Quickly find and verify data from various portfolios in centralized data repository.
Provide critical medical information to all stakeholders from a single source of truth.
Capture and log adverse event data from multiple sources, eliminating silos across safety, medical information, and CRM systems.
Enable stronger decision-making through configurable reports and dashboards based on actionable intelligence.
Leading the Conversation
EVERSANA and ArisGlobal Announce Strategic Partnership to Transform Drug Safety Automation and Advance Integrated Compliance Models
EVERSANA will leverage ArisGlobal’s end to end LifeSphere Safety platform to strengthen next generation commercialization services
The LifeSphere difference
Of Top 50 Biopharmas
80% of the top 50 biopharma companies are our clients
Time Savings
30% reduction in time to complete monitoring activities
Cost Savings
30% immediate cost savings potential via our advanced automation capabilities
Compliance
100% compliance with all present and upcoming regulatory standards
Learn more about our collaborative partnership with customers.

