An elegant solution to match, maintain, sync and integrate reference data, SPORIFY saves time, simplifies ongoing data management and harmonizes data across systems and geographies.
With SPORIFY your organization can:
- Generate an estimated 75% efficiency gains and time savings on initial data preparation and mapping
- Simplify ongoing data management activities with automatic data syncing and update notifications
- Prepare for IDMP implementation and enable interoperability across systems and geographies by managing regulatory compliant product data in a central, user-friendly interface
Discover the Benefits of SPORIFY for your organization
Central Location for IDMP compliance
Prepare and maintain compliant product data through real-time SPOR updates to keep pace with the phased implementation of ISO IDMP standards
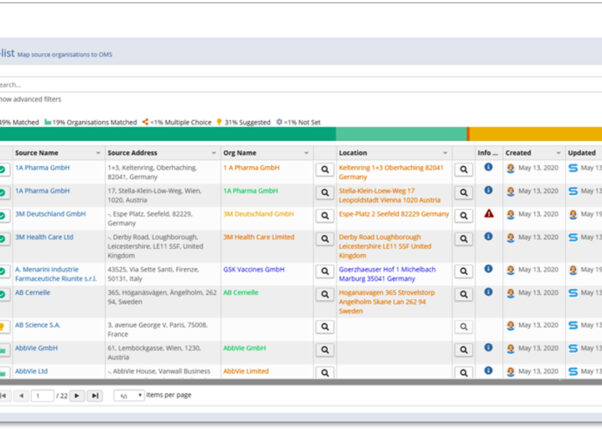
Easy RIM Integration
A vendor-agnostic application that connects via lightweight APIs, SPORIFY is compatible with any RIM system; there’s no need to migrate.
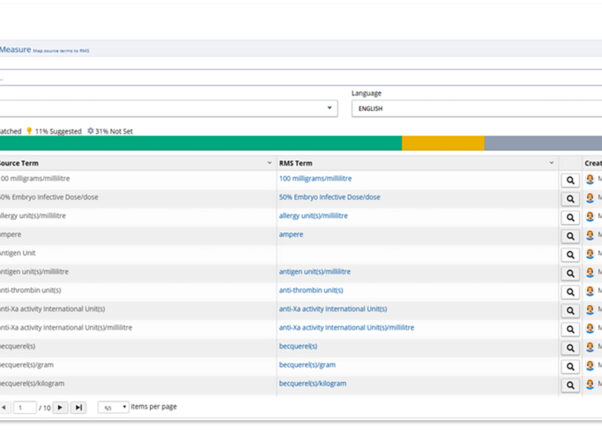
Increased Productivity and Time Savings
Complete initial data mapping up to 9X faster and use automated data synchronization to stay aligned with changes.
Seeing is believing
See SPORIFY in Action
The SPORIFY difference
SPORIFY is a trusted partner of leading pharmaceutical companies around the globe, including 5 of the world’s top 20 largest organizations. The technology also plays a vital role in facilitating data clean up, enrichment and harmonization for several European regulatory agencies, working groups and life sciences technology vendors. Here’s why SPORIFY is different:
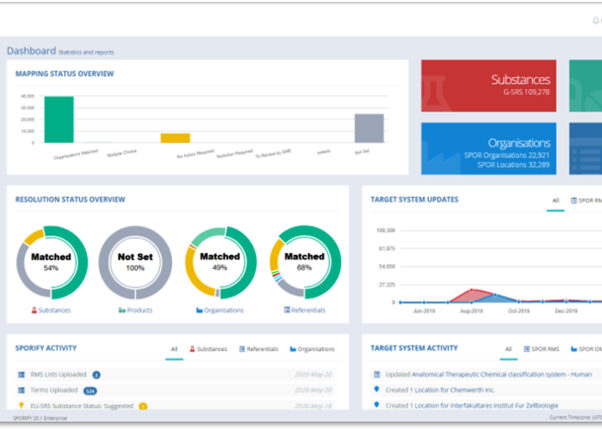
EMA SPOR mapping and integration
APIs provide a simple and secure solution to enable mapping, sync and notification services between your systems and SPOR master data.
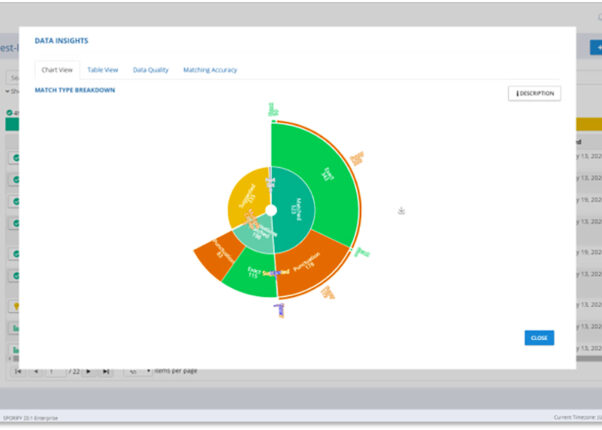
User-friendly data insights
Color-coded data insights that provide visibility to the latest SPOR values.
Automatic data sync and notifications
Real-time notifications when SPOR is updated for selected data elements of interest
Resources for Your Reference Data Management Journey
SPORIFY Product Fact Sheet
Discover the benefits of SPORIFY for reference data management in one place as an overview to stakeholders within your organization.
Top 5 Pharma Case Study
Read about the immediate productivity gains and compliance benefits a Global Regulatory Affairs team experienced by adopting the SPORIFY solution.
Pharma Tech Outlook Profile
SPORIFY was recognized as a Top Solutions Providers for 2023 in a recent issue of Pharma Tech Outlook for driving data-centric innovation for IDMP readiness.
Learn more about our collaborative partnership with customers